Reaction rate constant
In chemical kinetics a reaction rate constant k or 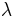 quantifies the speed of a chemical reaction.[1]
quantifies the speed of a chemical reaction.[1]
For a chemical reaction where substance A and B are reacting to produce C, the reaction rate has the form:
- Reaction: A + B → C
k(T) is the reaction rate constant that depends on temperature.
[C] is the concentration of substance C in moles per volume of solution assuming the reaction is taking place throughout the volume of the solution (for a reaction taking place at a boundary it would denote something like moles of C per area).
The exponents m and n are called orders and depend on the reaction mechanism. They can be determined experimentally.
A single-step reaction can also be written as
Ea is the activation energy and R is the Gas constant. Since at temperature T the molecules have energies according to a Boltzmann distribution, one can expect the proportion of collisions with energy greater than Ea to vary with e-Ea/RT. A is the pre-exponential factor or frequency factor.
The Arrhenius equation gives the quantitative basis of the relationship between the activation energy and the reaction rate at which a reaction proceeds.
Contents |
Units
The units of the rate coefficient depend on the global order of reaction:[2]
- For order n, the rate coefficient has units of mol1-n·Ln-1·s-1
- For order zero, the rate coefficient has units of mol·L-1·s-1
- For order one, the rate coefficient has units of s-1
- For order two, the rate coefficient has units of L·mol-1·s-1
Plasma and gases
Calculation of rate constants of the processes of generation and relaxation of electronically and vibrationally excited particles are of great importance. It is used for example, in the computer simulation of processes in plasma chemistry or microelectronics. First-principle based models should be used for such calculation. It can be done with the help of computer simulation software.
See also
References
- ^ http://www.chem.arizona.edu/~salzmanr/480a/480ants/chemkine.html
- ^ Blauch, David. "Differential Rate Laws". Chemical Kinetics. http://www.chm.davidson.edu/vce/kinetics/differentialratelaws.html.
![\frac{d[C]}{dt} = k(T)[A]^{m}[B]^{n}](/2012-wikipedia_en_all_nopic_01_2012/I/93de22176437c3c242cfd0c636e624e7.png)
![\frac{d[C]}{dt} = Ae^\frac{-E_a}{RT}[A]^m[B]^n](/2012-wikipedia_en_all_nopic_01_2012/I/a52b16dcc215ece18e7369a0add900ff.png)